Cases from the Medical College of Wisconsin
A mycotic aneurysm, retroperitoneal hematoma, and more.
Case 1: Persistent COVID-19 positivity
By Mark Ehioghae, MSc, ACP Medical Student Member; Harini Shah, BS, ACP Medical Student Member; Anu Taylor, MD, ACP Member; Brian Buggy, MD, FACP; Gabriel Mikhael, MD, FACP; and Devesh Kumar, BS, ACP Medical Student Member
The patient
A 43-year-old man with a history of mediastinal diffuse large B-cell lymphoma, in remission for two years after receiving chimeric antigen-receptor T-cell (CAR-T) therapy, presented with a persistent cough, myalgia, and a headache. Initial chest imaging, including X-ray and CT, appeared normal. An antigen test for SARS-CoV-2 was positive. The patient had previously received mRNA COVID-19 vaccinations (Moderna) at 13, 12, 11, and 6 months prior to presentation.

Despite initial mild symptoms, the patient's condition worsened. On day 21, he experienced a severe diffuse headache, which prompted a return to the hospital. Imaging studies, including CT of the head and MRI, showed mild cerebral edema and diffuse dural thickening. Cerebrospinal fluid analysis, however, revealed normal results with negative viral encephalitis panel and cytology. Blood tests indicated a total white blood cell count of 2,400 cells/µL (reference range, 4,200 to 11,000 cells/µL), with an absolute neutrophil count of 2,200 cells/µL (reference range, 1,800 to 7,700 cells/µL) and a total lymphocyte count of 500 cells/µL (reference range, 1,000 to 4,800 cells/µL). A polymerase chain reaction test for SARS-CoV-2 returned positive with a cycle threshold of 36.3.
Despite symptomatic treatment and the resolution of his headache by day 35, the patient's fever, chills, nonproductive cough, and dyspnea persisted. On day 51, a follow-up chest X-ray revealed bilateral ground-glass opacities, and pulse oximetry on room air at rest was 93%. Empiric treatment with azithromycin and ceftriaxone was initiated. Tests for bacterial, fungal, mycobacterial, and viral infections were negative. He was given posaconazole while awaiting 16s ribosomal DNA analysis for bacteria and mycobacteria with 28s ribosomal DNA for fungi, all of which were ultimately negative. The patient continued to test positive for COVID-19 with a cycle threshold of 34.5, and his total lymphocyte count remained low at 600 cells/µL. The patient declined remdesivir and despite a three-week course of prednisone, 40 mg/d, showed no clinical improvement.
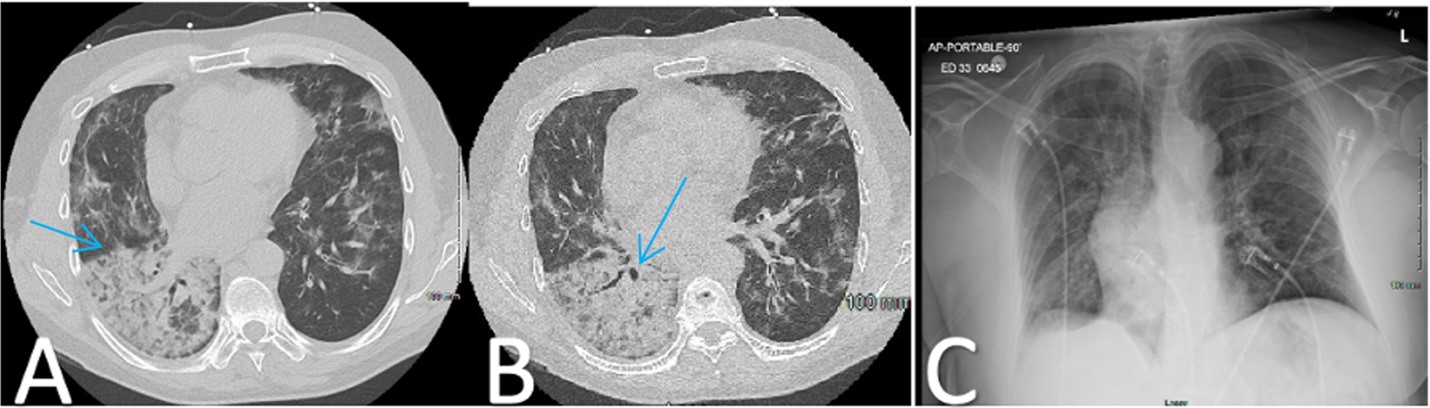
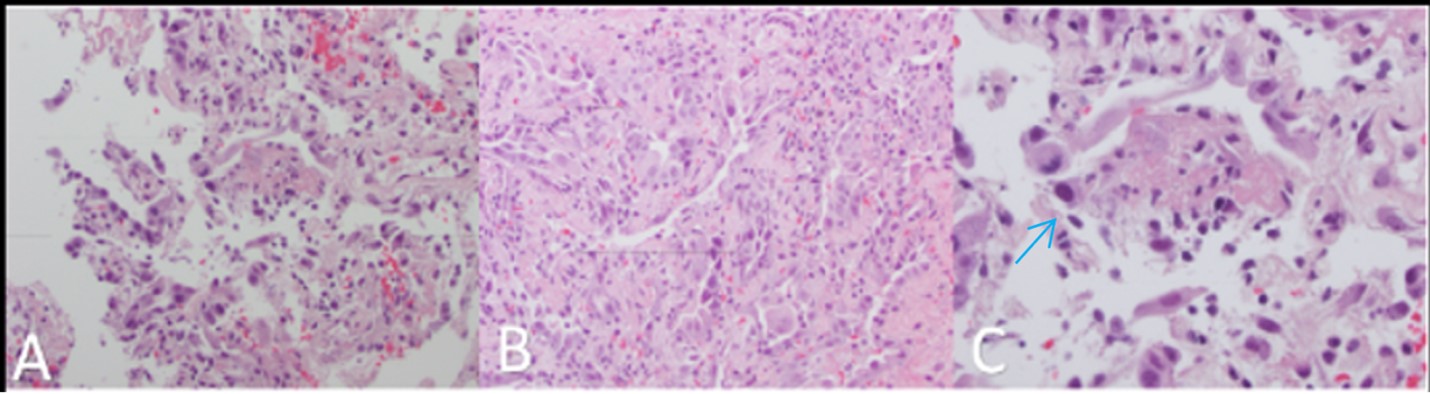
Two weeks later, on day 65, the patient returned to the hospital with worsening infiltrates on chest X-ray and CT (Figure 1), coupled with resting hypoxemia at 83%, which improved to 96% on 4 L of supplemental oxygen per minute. The cycle threshold for COVID-19 remained unchanged at 34.6. Bronchoscopy yielded negative results for bacterial, fungal, mycobacterial, and viral infections, except for a barely detectable Aspergillus galactomannan. The patient was again prescribed posaconazole while awaiting 16s ribosomal RNA analysis, which ultimately was negative. Transbronchial biopsy revealed diffuse alveolar damage with hyaline membrane formation (Figure 2).
The patient exhibited rapid clinical improvement following IV methylprednisolone and was placed on a prolonged (two-month) prednisone taper, leading to sustained clinical resolution of symptoms and clearance of infiltrates on chest X-ray.
The diagnosis
The diagnosis is an organizing pneumonia presenting due to prolonged COVID-19 positivity in an immunocompromised patient treated with CAR-T therapy. The prevalence of postacute sequelae of SARS-CoV-2 infection, also known as "long COVID," is not well defined. Estimates suggest that anywhere from 10% to 30% of individuals recovering from COVID-19 may experience prolonged symptoms. Treatment with CAR-T therapy appears to pose an elevated risk of severe COVID-19 and prolonged infection. Corticosteroid treatment has shown promise in managing severe COVID-19 complications, including organizing pneumonia, in immunocompromised individuals. However, standardized protocols for prolonged COVID-19 pneumonitis are lacking.
This case demonstrates that even nearly two years post-CAR-T therapy, persistent lymphopenia can hinder viral clearance, contributing to prolonged COVID-19 positivity. Additionally, the patient's positive Aspergillus result highlights extended infection risks in immunocompromised individuals.
Pearls
- Immunocompromised patients with hematological malignancies, especially those treated with CAR-T therapy, are at heightened risk for severe and prolonged COVID-19 infection.
- Corticosteroid treatment may be beneficial in managing severe COVID-19 complications in immunocompromised patients, but optimal timing and dosage should be carefully considered.
Case 2: Large mycotic aneurysm
By Nikita Piryani, BS, ACP Medical Student Member; Mark Ehioghae, MSc, ACP Medical Student Member; and Abhijai Singh, MD, ACP Member
The patient
A 74-year-old man with a history significant for chronic combined systolic and diastolic heart failure, coronary artery disease, peripheral artery disease, type 2 diabetes, end-stage renal disease on hemodialysis, unspecified pulmonary hypertension, and listeriosis of unknown origin presented with fever, shortness of breath, fluid overload, and fatigue. The patient was hypoxic (oxygen saturation, 91%), tachypneic (respiratory rate, 22 breaths/min), and febrile (100.8 °F).
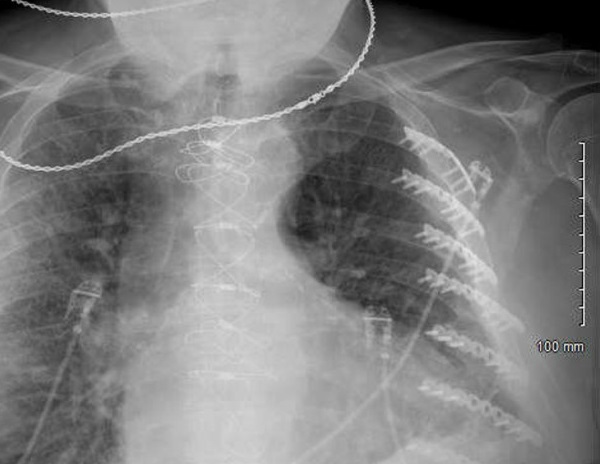
Chest X-ray revealed cardiomegaly with mild pulmonary congestion and possible consolidation in the right midlung (Figure 3). With an admitting diagnosis of community-acquired pneumonia, the patient was started on empiric antibiotics, including vancomycin, cefepime, and doxycycline.
Blood cultures confirmed the presence of Listeria monocytogenes. Given the patient's recurrent listeriosis and a previous adverse reaction to ceftriaxone, he was transitioned to linezolid. The patient's platelet counts were closely monitored as he had a history of thrombocytopenia while on the medication.
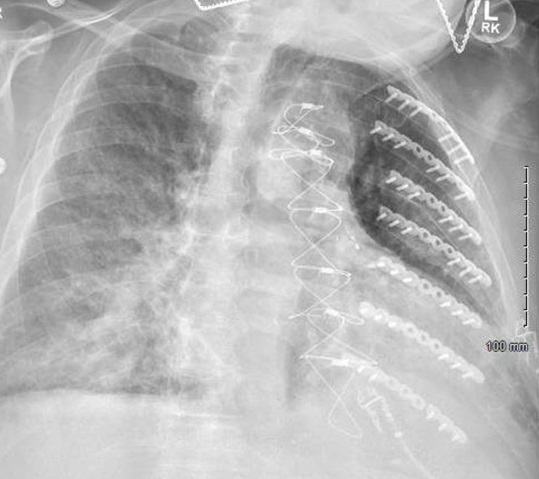
While on treatment, the patient developed increasing dyspnea and chest pain. A repeat chest X-ray showed new right lung opacities (Figure 4). Chest CT without contrast revealed a new short-segment dissection versus an intramural hematoma. CT angiography was deferred due to the patient's elevated creatinine level. A penicillin challenge was recommended due to concern of endovascular infection, and it was well tolerated. The patient was switched to IV ampicillin.
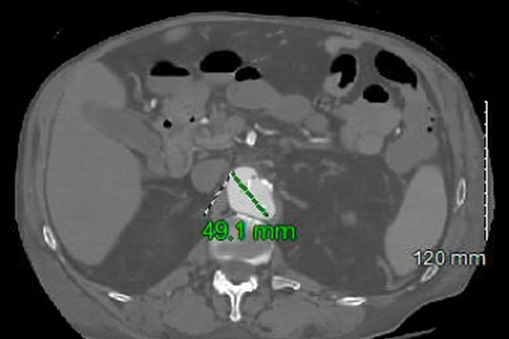
The patient reported new abdominal pain on the sixth day, and CT without contrast revealed retroperitoneal hemorrhage and thickening around the abdominal aorta. Stat CT angiography confirmed acute to subacute ulcers in the distal aortic arch and a 4.9-cm irregular ulcer in the suprarenal abdominal aorta (Figure 5). Due to the patient's comorbidities, surgical intervention was not recommended, and he was treated with six weeks of IV ampicillin. Dialysis and antibiotic lines were placed, and the patient received follow-up care from nephrology and physical/occupational therapy. After discharge, the patient continued home health care and outpatient dialysis.
The diagnosis
The diagnosis is a large mycotic aneurysm with retroperitoneal hematoma in the setting of Listeria bacteremia. Listeria monocytogenes does not typically cause serious illness in immunocompetent individuals. Approximately 1.3% of aortic aneurysms in developed countries are mycotic. Most are caused by gram-positive Staphylococcus, Enterococcus, Streptococcus pneumoniae, and Clostridium or gram-negative Salmonella species. Of the 18 cases of infectious aortitis cited in literature, only nine were abdominal aortic aneurysms. Listeriosis bacteremia leading to the rupture of an abdominal aortic aneurysm is an extremely rare finding. Risk factors for mycotic aneurysms include arterial injury, antecedent infection, impaired immunity, atherosclerosis, and pre-existing aneurysms. Numerous studies suggest that these conditions can result in vulnerable aortic walls, which increases the chances of developing a mycotic aneurysm. The bacteria can infiltrate the cardiovascular system in various locations, including the vasa vasorum of the arterial walls, vulnerable tunica intima, arteriosclerotic plaques, or thrombus material.
The standard course of treatment for mycotic aneurysm is combined antibiotic therapy with surgical debridement and revascularization. Surgical options include open repair or endovascular aneurysm repair. However, for patients who are not surgical candidates, antibiotic therapy alone is recommended. The antibiotic therapy is dependent upon the causative organism and should generally be continued for six weeks parenterally or orally.
Pearls
- Listeria monocytogenes does not typically cause serious illness in immunocompetent individuals, but clinicians should be aware of the risks in immunocompromised patients.
- Retroperitoneal hemorrhage in the setting of contained abdominal aortic aneurysm is rare, but early workup for possible endovascular complications is paramount.
Case 3: Retroperitoneal hematoma
By Chiemerie Ogbonnaya, BS, ACP Medical Student Member; Hari Anandarajah, BS, ACP Medical Student Member; and Abhijai Singh, MD, ACP Member
The patient
A 60-year-old man with a medical history significant for end-stage renal disease treated with hemodialysis three times a week, hypertension, coronary heart disease, peripheral artery disease, and hyperlipidemia presented with acute onset of left-sided abdominal and flank pain radiating to the chest and back. He reported shortness of breath and anuria but no cough, hematochezia, melena, or any other symptoms. The patient had recently been diagnosed with unprovoked right popliteal acute deep venous thrombosis and discharged on warfarin.
At ED presentation, the patient had tachycardia (110 beats/min) and hypertension (250/110 mm Hg). Workup was notable for a drop in hemoglobin level from 10.2 g/dL to 6 g/dL (reference range, 13.8 to 17.2 g/dL), a drop in hematocrit from 31% to 19% (reference range, 38.3% to 48.6%), and excessive anticoagulation with a supratherapeutic international normalized ratio (INR) of 4.3 (reference range, 0.8 to 1.2) indicative of acute blood loss and normocytic anemia. The patient was hyperkalemic at 5.5 mmol/L (reference range, 3.5 to 5 mmol/L), with elevations in creatinine level at 14.06 mg/dL (reference range, 0.6 to 1.2 mg/dL) and blood urea nitrogen level at 67 mg/dL (reference range, 6 to 20 mg/dL).
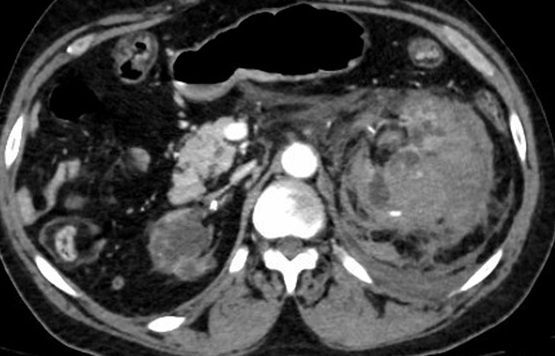
A CT of the abdomen/pelvis revealed a moderate-to-large left-sided retroperitoneal hematoma secondary to a hemorrhagic left kidney with multiple foci of active extravasation (Figure 6). No clear solid mass had been identified on prior imaging, and the hemorrhage was presumed to result from spontaneous cyst rupture due to acquired polycystic renal disease consistent with end-stage renal disease. The patient was diagnosed with class II hemorrhagic shock with moderate thrombotic thrombocytopenic purpura and was admitted to the ICU.
Warfarin was discontinued upon ICU admission, and INR was corrected with vitamin K and prothrombin complex concentrate. Resuscitation consisted of four units of fresh frozen plasma, two units of platelets, and one unit of packed red blood cells. The patient was also started on IV nicardipine for hypertension. After conservative management, his hemoglobin stabilized and he started apixaban without any issues.
The patient was discharged to a nursing facility in stable condition on apixaban and sevelamer carbonate, with symptomatic improvement including resolution of hyperkalemia and thrombocytopenia.
The diagnosis
The diagnosis is a moderate-to-large left-sided retroperitoneal hematoma. A retroperitoneal hematoma is characterized by abdominal or lower back pain (present in 64% of patients) and suprainguinal tenderness (present in 100%). The severity of these symptoms can range from mild discomfort to intense pain. In severe cases, hypotension and signs of shock may be present due to significant blood loss. Causes of spontaneous retroperitoneal hematomas vary, including trauma, anticoagulants, and vascular conditions, with the most common cause being tumors. Renal cell carcinoma and angiomyolipoma are the most common tumors, occurring in 57% to 73% of cases. Diagnosis of retroperitoneal hematomas relies on clinical evaluations and diagnostic tools, with CT scans being the gold standard. Early diagnosis and management are essential in addressing this condition and its underlying causes.
Pearls
- Patients on anticoagulants, such as warfarin, are at increased risk of developing a retroperitoneal hematoma.
- Early diagnosis of retroperitoneal hematomas through workup, including imaging, is crucial for the best prognosis.
Case 4: Disseminated blastomycosis
By Natalie Anumolu, BS, ACP Medical Student Member; Jack Geiger, DO; Narendra Veerapaneni, MD, FACP; and Pinky Jha, MD, MPH, FACP
The patient
A 47-year-old man with a history of latent tuberculosis, disseminated blastomycosis (treated with six months of itraconazole), and chronic hepatitis B (on entecavir) but no documented history of immunosuppression presented with hemoptysis, cough, and fever in May 2022 to a hospital in the Midwestern U.S.
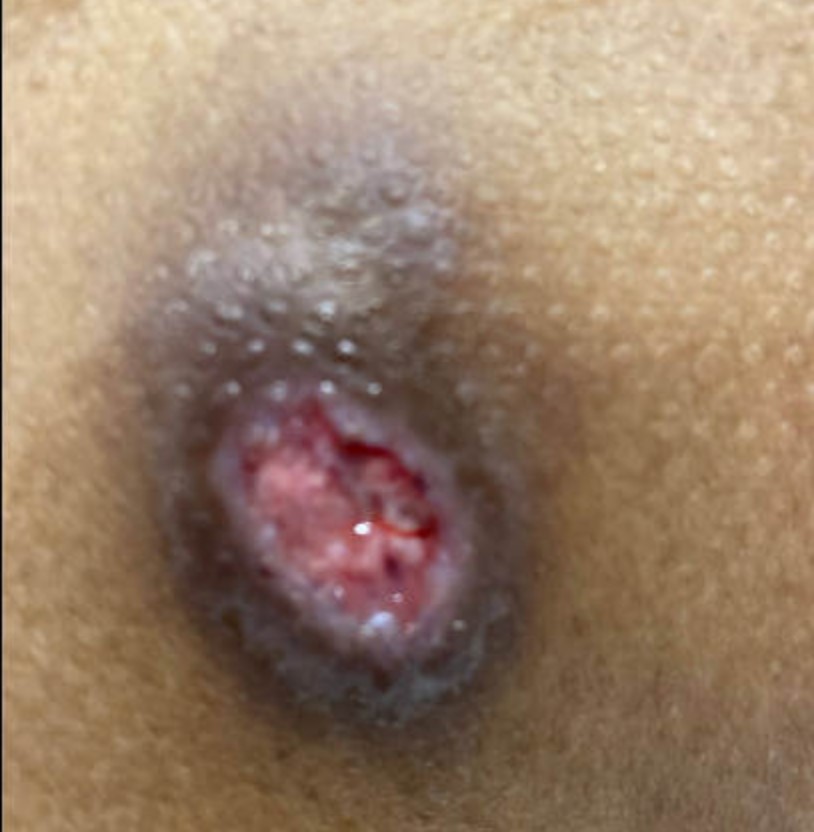
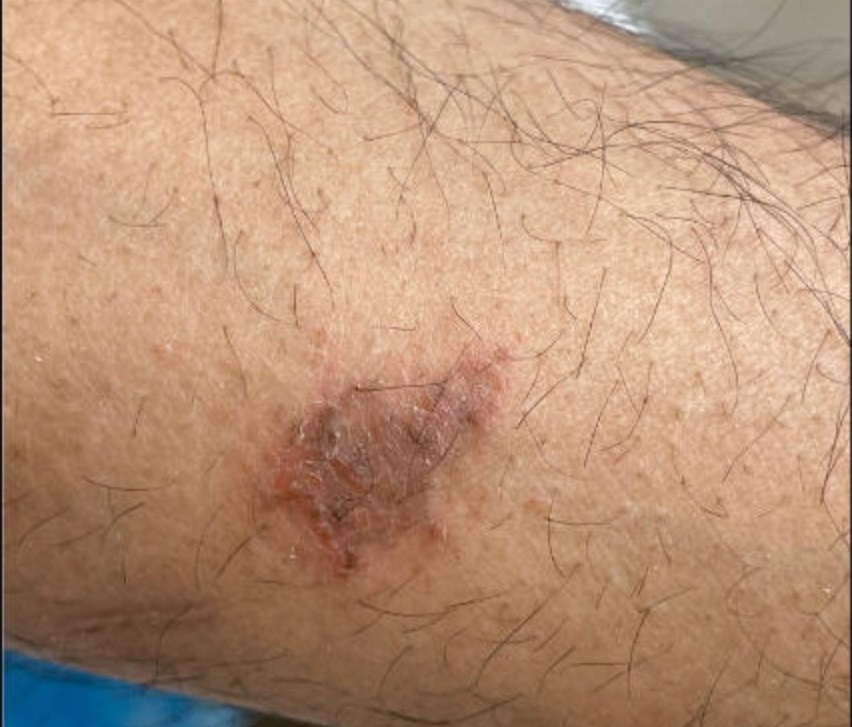
In May 2022, the patient's bronchoscopy with bronchoalveolar lavage was suspicious for blastomycosis, blastomycosis antigen was positive, and a biopsy from a necrotic ulcer on his lower back confirmed blastomycosis (Figure 7). He completed six months of itraconazole and was referred to an infectious disease clinic. At an appointment in February 2023, the patient had a new skin lesion on his left forearm (Figure 8) and reported chest discomfort. A skin sample from biopsy was scant, and no fungal elements were seen.
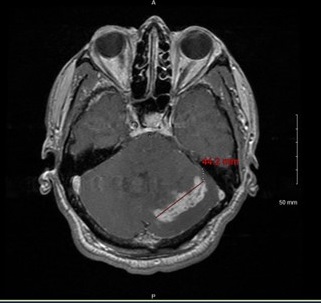
In August 2023, the patient presented with a one-week history of persistent worsening bilateral headaches refractory to over-the-counter medication, dizziness, nausea, vomiting, and ataxia. CT and MRI imaging revealed a heterogeneous enhancing left cerebellar mass with adjacent edema and effacement of the fourth ventricle (Figure 9).
The patient was treated with IV liposomal amphotericin B, then transitioned to oral voriconazole, which was expected to be continued for 12 months.
The diagnosis
The diagnosis is central nervous system (CNS) blastomycosis. Blastomycosis is a fungal infection endemic to the Ohio and Mississippi River valleys and the Great Lakes regions, caused by Blastomyces dermatitidis. True prevalence and incidence are unknown as blastomycosis is not a reportable infection; furthermore, up to 50% of infections are subclinical. However, incidence is estimated to be less than 1 case per 100,000 people. Symptomatic patients typically present with pulmonary symptoms, as Blastomyces spores enter the host through inhalation. In 20% to 50% of cases, blastomycosis is reported to disseminate. In cases of dissemination, 40% to 80% patients develop cutaneous manifestations. Bone is the second most common site of dissemination, occurring in 5% to 25%. CNS involvement is estimated to occur in 1% to 10% of blastomycosis cases, typically presenting in the context of disseminated disease. Reports of CNS blastomycosis are limited; one study published in January 2023 found 25 cases of CNS blastomycosis in the literature.
CNS blastomycosis can present as meningitis, encephalitis, or an abscess; intracranial lesions can exist as single or multiple lesions and frequently involve the cerebellum. For diagnosis, culture is the gold standard; however, cerebrospinal fluid (CSF) cultures for blastomycosis have poor sensitivity. While most patients with CNS blastomycosis are identified through imaging, imaging is not diagnostic as CNS blastomycosis appears similar to other fungi. Instead, tissue biopsy can confirm blastomycosis. Current Infectious Diseases Society of America guidelines for treatment of CNS blastomycosis call for four to six weeks of IV amphotericin B followed by 12 months of oral azole therapy.
Pearls
- Include blastomycosis on the differential for CNS disease, especially in the Midwestern U.S.
- Long-term follow-up and monitoring with infectious diseases subspecialists are important even when blastomycosis treatment is complete and patients show no obvious signs of immunosuppression.
Case 5: Pancytopenia secondary to Epstein-Barr virus
By Nana Danso, BS, ACP Medical Student Member; Hari Anandarajah, BS, ACP Medical Student Member; Antoni Wojtkowski, MD, FACP; and Brian Quinn, MD, FACP
The patient
An 18-year-old man with no significant medical history presented with four days of worsening fever, sore throat, nausea, vomiting, and difficulty swallowing. One month prior, he had presented to urgent care with a tender lump below his chin and lymphadenopathy. He was diagnosed with infectious mononucleosis and discharged with a one-week course of prednisone. Two days prior to his current presentation, the patient was evaluated at an ED and diagnosed with viral pharyngitis. At that time, he declined group A streptococcus and monospot testing.
On the day of this admission, the patient presented to an ED with fever of 103 °F, tachycardia of 135 beats/min, and a positive monospot test. Workup was notable for a white blood cell count of 500 cells/µL (reference range, 4,500 to 11,000 cells/µL), hemoglobin level of 8.8 g/dL (reference range, 14 to 18 g/dL), absolute neutrophil count of 10 cells/μL (reference range, 1,500 to 8,000 cells/μL), and platelet count of 6,000/μL (reference range, 150,000 to 450,000/μL), prompting transfer to our institution. The patient was hyponatremic with a sodium level of 131 mmol/L (reference range, 136 to 145 mmol/L), likely due to poor intake, and had acute kidney injury, with a creatinine level of 1.4 mg/dL (reference range, 0.7 to 1.3 mg/dL), but no transaminitis. He was positive for Epstein-Barr virus (EBV) IgM and IgG. Tests for adenovirus, COVID-19, cytomegalovirus, hepatitis B, hepatitis C, HIV, group A streptococcus, direct antiglobulin, paroxysmal nocturnal hemoglobinuria, histoplasma, and Blastomyces were negative. Abdominal ultrasound showed no hepatosplenomegaly. Chest X-ray was unremarkable.
The patient was started on cefepime and switched to levofloxacin, fluconazole, and acyclovir for neutropenic fever prophylaxis. Immune thrombocytopenia was judged unlikely as the platelet count was not affected by four days of dexamethasone, 40 mg/d, but did improve with two irradiated units of platelets. He received several days of filgrastim, a granulocyte colony-stimulating factor agonist, and eltrombopag, a thrombopoietin-receptor agonist, to stimulate bone marrow recovery.
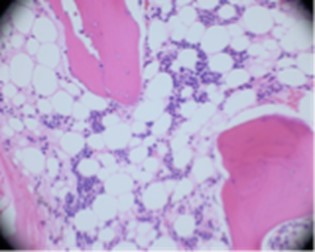
Prolonged pancytopenia prompted bone marrow biopsy, which revealed hypocellular marrow with trilineage hypoplasia, negative for a hematolymphoid neoplasm (Figure 10). These findings suggested marrow recovery following an EBV-associated aplastic anemia. A CT of the head/neck was performed on day five to rule out neck abscess given dysphagia and leukopenia. Imaging revealed enlarged tonsils, likely secondary to mononucleosis, as well as possible bronchitis.
The patient was discharged after symptomatic improvement on both cyclosporine, 200 mg twice daily, and eltrombopag, 150 mg/d for 30 days, for full blood count recovery and continued infection prophylaxis. He responded favorably and avoided bone marrow transplant.
The diagnosis
This diagnosis is EBV infectious mononucleosis, with the rare complication of severe pancytopenia and neutropenic fever. EBV is a common infection that affects 95% of the human population at some point. It is usually asymptomatic, but in adolescents and young adults, it can result in mononucleosis. In the U.S., the annual incidence for infectious mononucleosis ranges from 11 to 48 cases per 1,000 persons. Patients commonly have sore throat, fatigue, myalgias, and lymphadenopathy but can also experience splenomegaly, nausea, vomiting, palatal petechiae, and rash. While mild neutropenia has been associated with EBV, pancytopenia is a rare presentation in immunocompetent patients and has potentially fatal consequences. Our patient presented with a severity of thrombocytopenia rarely seen with EBV bone marrow suppression. There is currently no effective antiviral treatment for EBV infectious mononucleosis in immunocompetent patients. Although acyclovir and ganciclovir may reduce EBV shedding, they are clinically ineffective. EBV mononucleosis with hemolytic anemia and thrombocytopenia is typically treated with short courses of corticosteroids, but this treatment is not indicated for uncomplicated cases. Pancytopenia is typically treated with bone marrow-stimulating drugs.
Pearls
- Diagnosing EBV infectious mononucleosis can be challenging when patients present with pancytopenia and neutropenic fever.
- A thorough workup with biopsy and viral serology, as well as early detection and treatment with bone-marrow stimulating drugs, are crucial to prevent superinfections and ensure a prompt recovery from pancytopenia.
Case 6: Drug-induced aseptic meningitis
By Raquel Valdes, BA, ACP Medical Student Member; Jasmine Garcia, BS, ACP Medical Student Member; Melissa Weekes, MD, ACP Member; and Devin Madenberg, DO, FACP
The patient
A 56-year-old woman with a history of hypertension, hyperlipidemia, and idiopathic polyneuropathy presented with high-grade fevers, headache, neck pain/stiffness, and photophobia that started within hours of her fourth IV immunoglobulin (IVIG) infusion. The IVIG infusions had been started two weeks prior for her polyneuropathy. After her second infusion, she had developed similar but less severe symptoms that resolved within one day. On this admission, she was febrile (102.6 °F) and tachycardic. Labs were unremarkable, without leukocytosis or lactic acidosis. A CT of the head and a chest X-ray were unremarkable. She was started on empiric vancomycin, ceftriaxone, and acyclovir for presumed meningitis. Shortly thereafter, she underwent a lumbar puncture. The meningitis/encephalitis panel was negative, protein and glucose CSF levels were normal, and the CSF white blood cell count was 359 cells/µL with 42% neutrophils/51% lymphocytes/7% monocytes. CSF cultures were negative, and antibiotics were discontinued. Within 48 hours, her symptoms completely resolved, and she was discharged.
The diagnosis
The diagnosis is drug-induced aseptic meningitis secondary to IVIG. Aseptic meningitis is defined as meningeal inflammation with negative bacterial Gram stain/culture and can be attributed to a variety of infectious or noninfectious causes. Infectious causes are the most common, with viral etiologies accounting for around 62% of cases. Of the viral causes, enteroviruses account for around 51.6%, followed by herpes simplex virus in 8.3% of cases. Other infectious causes of aseptic meningitis include bacterial (Mycobacterium tuberculosis, Treponema pallidum), fungal (Candida, Cryptococcus, Histoplasma), or parasitic (Toxoplasma, Trichinella) infections. Noninfectious causes can include malignancies (lymphoma, leukemia), paraneoplastic conditions, autoimmune diseases (sarcoidosis, Sjögren's, systemic lupus erythematosus), or medications.
The incidence of drug-induced aseptic meningitis is unknown, but it is thought to be a relatively uncommon adverse reaction to certain medications. In addition to IVIG, NSAIDs, vaccines, and antimicrobials such as trimethoprim/sulfamethoxazole have also been associated with aseptic meningitis. Only around 40 to 50 cases of IVIG-induced meningitis are known, although the condition is probably underreported. Risk factors for drug-induced aseptic meningitis with IVIG include a history of migraines and higher IVIG doses, although it can occur at any dose. Symptoms usually manifest after six hours but within 48 hours of infusion.
Supportive management with analgesia, antipyretics, and antiemetics is the mainstay of treatment, and symptoms usually improve within 48 to 72 hours of IVIG cessation. However, drug-induced aseptic meningitis is a diagnosis of exclusion, and typical presenting symptoms such as headache and fever are nonspecific. CSF analysis is therefore extremely important in ruling out other etiologies such as pyogenic bacterial meningitis. After drug-induced aseptic meningitis from IVIG is diagnosed, informed discussions should be had regarding future treatments, including stopping IVIG therapy, trialing other IVIG brands, trialing subcutaneous IVIG, and/or starting premedications like corticosteroids or antihistamines.
Pearls
- Aseptic meningitis is a challenging diagnosis that requires ruling out potentially deadly pathogens.
- Drug-induced aseptic meningitis is uncommon but can occur due to many different classes of medications, including IVIG, NSAIDs, vaccines, and antimicrobials.



