Sumatriptan-induced polymorphic ventricular tachycardia
A patient with atypical chest pain and negative stress tests suddenly became pulseless.
The patient
A 49-year-old woman with a history of migraine (treated with sumatriptan for more than 15 years, 50 mg taken orally), rheumatoid arthritis, and hypothyroidism presented with two hours of right-sided pleuritic chest pain that radiated into her right neck and back between the scapulae. It was not related to exertion but did improve with nitroglycerin. She reported associated dyspnea and lightheadedness. Her chest pain was atypical and considered low-risk. Physical exam was negative for any abnormalities. Her father had coronary artery disease, but she otherwise had no risk factors for cardiovascular disease and did not report tobacco or illicit drug use. She had a previous episode of chest pain and had undergone exercise stress testing one year earlier, which was negative for ischemia.

On current presentation, troponin I level was elevated at 0.98 ng/mL (reference range ≤0.15 ng/mL). An electrocardiogram showed no ischemic changes. Urine drug screen was negative. Pharmacological stress testing with regadenoson was negative for ischemia. The patient reported her typical migraine pain and was given sumatriptan, 6 mg subcutaneously. Twenty minutes later, she became diaphoretic and tachycardic. Blood pressure was 124/84 mm Hg. She suddenly lost consciousness and became pulseless. Ventricular tachycardia was seen on the monitor.
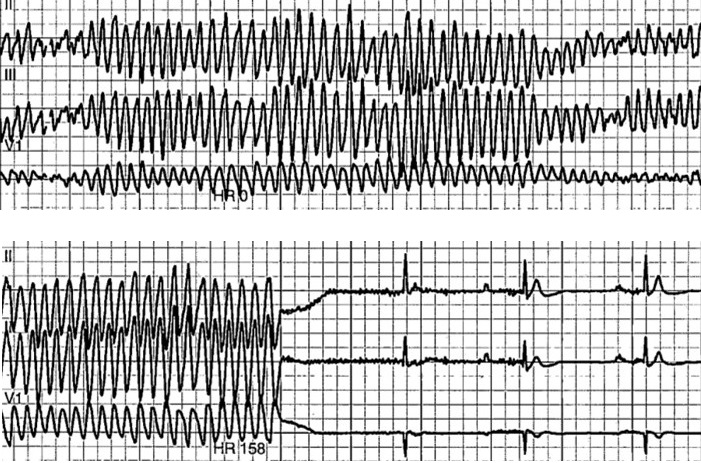
Return of spontaneous circulation was achieved after approximately 30 seconds of chest compressions, without medications, and telemetry demonstrated polymorphic ventricular tachycardia (Figure). Laboratory values included a potassium level of 3.3 mmol/L (reference range, 3.5 to 5.1 mmol/L), a magnesium level of 1.9 mg/dL (reference range, 1.8 to 2.4 mg/dL), a phosphorus level of 2.9 mg/dL (reference range, 2.5 to 4.9 mg/dL), an albumin level of 2.9 g/dL (reference range, 3.4 to 5.0 g/dL), and a corrected calcium level of 8.98 mg/dL (reference range, 8.5 to 10.1 mg/dL). Transthoracic echocardiogram showed an ejection fraction of 55% to 60%, grade 1 diastolic dysfunction without signs of regional wall motion abnormalities, no significant valvular disease, and no pericardial effusion. The patient subsequently underwent left-heart catheterization, which showed 40% to 50% stenosis of the proximal left anterior descending artery. An implantable cardioverter-defibrillator was placed prior to discharge. With regular outpatient follow-up, she has had no further episodes of arrhythmia.
The diagnosis
The diagnosis is sumatriptan-induced polymorphic ventricular tachycardia and cardiac arrest secondary to triptan-induced coronary vasospasm. The patient's history of previous nonischemic chest pain, lack of obstructive coronary artery disease, and response to nitroglycerin were consistent with coronary vasospasm. Her symptoms were exacerbated by triptan use, which is associated with coronary vasospasm secondary to activation of 5HT receptors and QTc prolongation, both of which predispose patients to arrhythmias.
The association between triptans and clinically significant cardiovascular vasospasm highlights the importance of screening patients for cardiovascular disease before using this class of medications. The American Headache Society recommends avoidance or cautious use of triptans in patients with coronary artery disease, peripheral vascular disease, uncontrolled hypertension, or other vascular risk factors. Life-threatening arrythmias, including ventricular tachycardia and ventricular fibrillation, occur in between 1 in 100 and 1 in 1,000 patients. Triptan use is contraindicated in patients with ischemic coronary artery disease, coronary vasospasm, and Wolff-Parkinson-White syndrome or any arrhythmias associated with cardiac accessory conduction pathway disorders. Due to the potential vasospastic action of serotonin agonists such as triptans, prescription of multiple serotonergic agents should be avoided. Clinicians should hold triptans and consider them as a contributory factor in patients hospitalized for chest pain and undergoing workup for acute coronary syndrome.
Prior to initiation of triptan therapy, cardiovascular risk factors (such as hypertension, hyperlipidemia, diabetes, sedentary lifestyle, obesity, family history, and use of tobacco or illicit drugs) should be reviewed and atherosclerotic cardiovascular disease risk should be calculated to reduce risk of coronary vasospasm and potentially fatal cardiac arrhythmia and ischemia. Patients who are low risk (<10%) can be started on triptan therapy whereas those at intermediate risk (10% to 20%) should try alternative therapies until cardiac evaluation rules out significant disease. High-risk (>20%) patients should avoid triptans entirely.
Pearls
- Before triptan therapy is started, cardiovascular risk factors and medications should be reviewed to risk stratify patients and reduce risk of coronary vasospasm as well as potentially fatal cardiac arrhythmia and ischemia.
- Triptans should be held and considered as a possible contributory factor in patients hospitalized for chest pain and undergoing workup for acute coronary syndrome.
